
While the positioning of the triple therapy recommended by GOLD, and by the FDA and EMA, is straightforward, the published evidence on which these recommendations are based suggest that there are other conditions in which triple might be considered or not recommended. The position proposed by the 2019 Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy document recommends triple therapy only as a step-up from LAMA/LABA or ICS/LABA in patients whose disease is not adequately controlled by one of these two combinations. Neither the European Medicines Agency (EMA) nor the US Food and Drug Administration (FDA) make a distinction between initiation of therapy in untreated newly diagnosed patients, versus use of triple therapy in already treated patients, as both assume triple therapy is a step-up. These slightly different indications have in common that triple therapy is recommended for patients not adequately controlled by existing inhaled combination therapies. In the USA, only FLF/VI/UMEC is approved, both for the long-term, maintenance treatment of airflow obstruction in patients with severe COPD and to reduce exacerbations of COPD in patients with a history of exacerbations. In addition, triple therapy might improve activity levels through bronchodilation or reduced breathlessness, and thereby improve respiratory muscle strength and impact upon disease progression.įLF/VI/UMEC is approved in the European Union as a maintenance treatment in adult patients with moderate-to-severe COPD who are not adequately treated by a combination of an ICS/LABA or LABA/LAMA, whereas BDP/FF/G is approved only for patients not controlled by LABA/ICS combination (the approval in addition to LABA/LAMA is pending). However, we may speculate that the simultaneous delivery to the target organ of three agents with different mechanisms of action may improve positive interactions between them. įor the time being, the additional benefit of a fixed triple LABA/LAMA/ICS combination is related to convenience for the patient, and possibly improved compliance. The two triple therapies were equally effective, and both superior to tiotropium alone.
In fact, there is only one study where the triple therapy in a single inhaler was compared to triple therapy in separate inhalers, but the LAMA was different with glycopyrronium in the former (BDP/FF/G) and tiotropium in the latter.
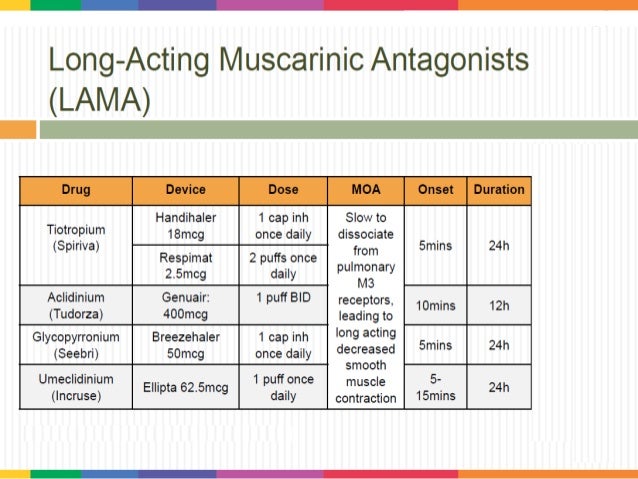
Whether triple therapy in a single inhaler outperforms the three individual components given in separate inhalers is unknown. The triple therapies available in a single inhaler are: beclomethasone-dipropionate/formoterol/glycopyrronium (BDP/FF/G) fluticasone-furoate/vilanterol/umeclidinium (FLF/VI/UMEC) and budesonide/glycopyrronium/formoterol (B/G/F).
#Lama drugs registration
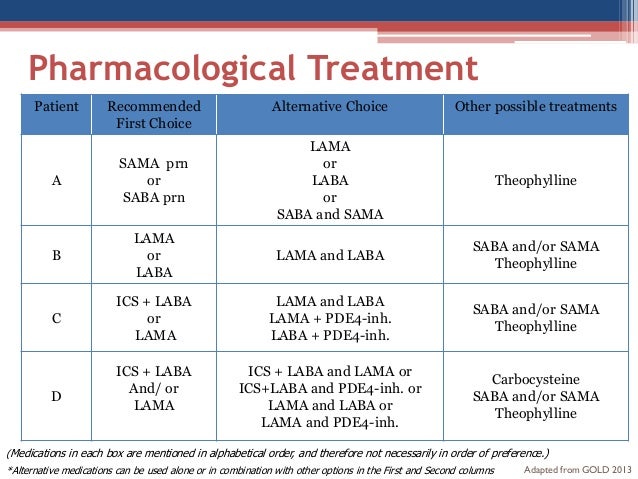
Triple inhaler therapy in COPD might in some real-life situations be useful outside of the strict indications reported by the registration agencies, but at the same time in some other situations it could be better avoided, even when recommended Ī current hot topic in COPD is that two “fixed triple” combinations of an inhaled corticosteroid (ICS), a long-acting β 2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) in a single inhaler have become available for patients with COPD, and a third triple therapy is in advanced development with the first large randomised clinical trial (RCT) recently published in Lancet Respiratory Medicine.


 0 kommentar(er)
0 kommentar(er)
